ir spectrum hcl
Each peak differentiating between 35Cl and 37Cl is assigned. The etalon and the interfering air absorption lines are included in the.
.jpg)
Analyzing The Gas Phase Spectrum Of Hydrogen Chloride With Ft Ir
FTIR Spectrometer Fourier-transform spectroscopy is a beautiful application of a scanning Michelson interfer-ometer.

. Energy transitions from the spectra were plotted vs. Location HCl spectrum DCl spectrum Mirror. The value for absorption is usually in cm -1.
The Infrared and Raman Spectroscopic Signals of HF HCl HBr and HI. Note that not all frequencies have a related compound. IR Spectrum Table by Frequency Range.
The IR range of the spectrum extends from 1 m the long-wavelength end of the visible region to 1000 m in the microwave region. Internet Electronic Journal of Molecular Design20032. 15 minutes 10 ml D.
Im IR-Spektrum liegen einige Oberschwingungen im normalerweise regi-strierten Bereich 4000 - ca. Cell opened which produced a violent reaction. Infrarotspektroskopie kurz IR-Spektroskopie und bis in die 1960er-Jahre Ultrarotspektroskopie genannt ist ein physikalisches Analyseverfahren das mit infraroter Strahlung Wellenlänge.
The High Resolution Infrared Spectrum of HCl The High Resolution Infrared Spectrum of HCl Authors J L Doménech 1 B J Drouin 2 J Cernicharo 3 V J Herrero 4 I Tanarro 4 Affiliations 1 Molecular Physics Department Instituto de Estructura de la Materia IEM-CSIC Serrano 123. Frequency from which several physical constants were determined. The IR Spectrum Table is a chart for use during infrared spectroscopy.
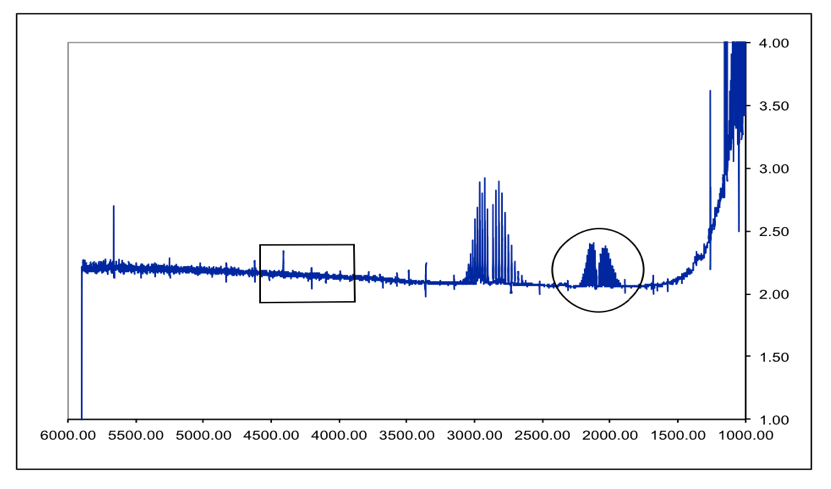
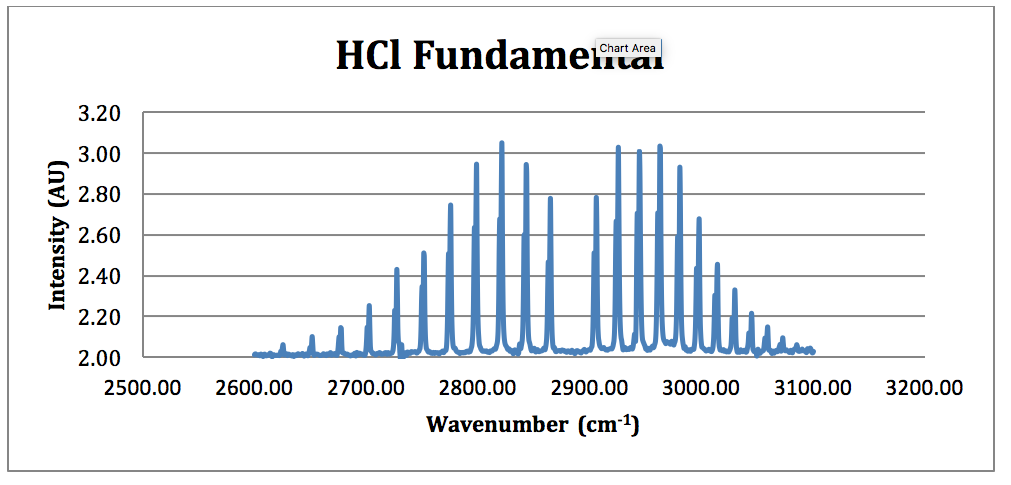
The free spectral range of the etalon is typically 2328 cm 1 7080 GHz which matches well with the 125 mm thickness of the IR quartz sample cell windows. A classic among molecular spectra the infrared absorption spectrum of HCl can be analyzed to gain information about both rotation and vibration of the molecule. Does this have something to do with fact that peaks in IR spectroscopy represent areas of the spectrum where specific bond vibrations occur and there.
Suggest one reason why HCl has only one peak. There were two branches that were apparent in the result of the spectroscopy the R branch and the P branch that correspond to J 1 and J -1 respectively. The table lists IR spectroscopy frequency ranges appearance of the vibration and absorptions for functional groups.
The gas ideally dried by passing it through a drying agent and is drawn into a gas sample cell. 𝜈 a 0 a 1 m Use a residual plot to show that it is necessary to add a term a 2 m2to the model. In this experiment we measure the infrared IR vibrational spectrum of a linear diatomic HCl molecule in the gas phase with rotational resolution ie with the rotational fine structure.
The HCl gas is usually prepared by the reaction of NaCl with H SO. Cell was closed detached and placed in the infrared spectrophotometer for analysis. D OM EN EC H Molecular Physics Department Instituto de Estructura de la Materia IEM-CSIC Serrano 123.
Stack Exchange network consists of 180 QA communities including Stack Overflow the largest most trusted online community for. The chlorine isotope peaks are resolved to baseline. There are two tables grouped by frequency range and compound class.
Gas Collection Once reaction was complete the IR. Spectra were collected on a Nicolet Nexus 670 FR-IR in a 10-cm gas cell at a pressure of 20 Torr. FTIR spectroscopy was used to analyze rotational-vibrational transitions in gas-state HCl and DCl and their isotopomers due to 35 Cl and 37 Cl to determine molecular characteristics.
The sample cell is placed in the sample chamber of the spectrometer and. The absorption lines shown involve transitions from the ground to first excited vibrational state of HCl but also involve changes in the rotational state. Vibration-Rotation Spectrum of HCl Add annotation to spectrum.
800 nm bis 1 mm arbeitet. The chloroniumyl cation HCl has been recently identified in space from Herschelʼs spectra. A joint analysis of extensive vis-UV spectroscopy emission data together with a few high-resolution and high-accuracy millimeter- wave data provided the necessary rest frequencies to support the astronomical identification.
The absorption lines shown involve transitions from the ground to first excited vibrational state of HCl but also involve changes in the rotational state. The rotational angular. The isotopic effect was observed in a spectrum of both HCl and DCl with DCl at a lower wavenumber than HCl which coincided with 37 Cl being observed at a lower frequency than 35 Cl.
Measure the transmission of an infrared light beam passing through a cell containing HCl vapor. The spectra of HCl and. 600 cm-1 und können daher mit zur Identifizierung herangezogen werden.
VIBRATION-ROTATION SPECTROSCOPY OF HCl By. Fourier transform infrared spectroscopy was used to study the vibrational and rotational motions of diatomic molecules hydrogen chloride HCl and deuterated chloride DCl. The major part of this assignment is an analysis of the infrared vibration-rotation spectrum of HCl in terms of the theoretical model discussed above First try a regression fit of the equation.
Produce sufficient amounts of HClDCl gas The system was evacuated for approx. The absorption spectrum will show dis-tinct absorption peaks whose frequencies and strengths will be compared with predictions. Zusätzlich gibt es auch noch sogenannte Kombi- Freie Universität Berlin - Institut für Chemie und Biochemie - Organisch-chemische Grundausbildung nationsschwingungen die dadurch zustande kommen.
John Ricely Abstract Using the Nicolet 6700 spectrometer the spectrum for HCl was analyzed. As illustrated in Fig. HCl and DCl IR absorption spectra Datasets PDF files of peak-labeled high resolution.
To use an IR spectrum table first find the frequency or compound in the first column depending on which type of chart you are using. A classic among molecular spectra the infrared absorption spectrum of HCl can be analyzed to gain information about both rotation and vibration of the molecule. 1 an IR beam.
Das Verfahren gehört zu den Methoden der Molekülspektroskopie die auf der Anregung von Energiezuständen in Molekülen beruhen. THE HIGH RESOLUTION INFRARED SPECTRUM OF HCl J. Recording the spectrum The proper way to use the Fourier Transform Infrared Spectrometer will be explained in the laboratory.
These constants were then used to determine the. Then find the corresponding values for absorption appearance and other attributes. O was added to flask F with the IR.

Ft Ir Spectra Of A Fexofenadine B A Mixture Of Fexofenadine And R Download Scientific Diagram

Measured Hcl Spectrum At The Mid Wave Infrared Notice The P And R Download Scientific Diagram

Infrared Spectrometric Rotational And Vibrational Analysis Of Hcl And Dcl Caroline Frank

Ftir Spectra Of Ciprofloxacin Hydrochloride Download Scientific Diagram

Ft Ir Spectra Of Lidocaine Hcl Hp B Cd 1 1 And 1 2 Lidocaine Download Scientific Diagram

Experiment 9 Rotational Vibrational Spectroscopy Introduction

Ir Spectrum Of Gemcitabine Hcl Download Scientific Diagram

Ir Spectrum Of Gemcitabine Hcl Download Scientific Diagram

Ketamine Standard Ftir Spectra Download Scientific Diagram
Spectroscopy And Molecular Structure Hci Dci

Datei Ir Hcl Rot Vib Mrtz Svg Wikipedia

Infrared Spectrometric Rotational And Vibrational Analysis Of Hcl And Dcl Caroline Frank

Datei Ir Hcl Rot Vib Mrtz Svg Wikipedia

Ftir Spectra Of Ondansetron Pure Drug Download Scientific Diagram
Comments
Post a Comment